Organ Regeneration Platform
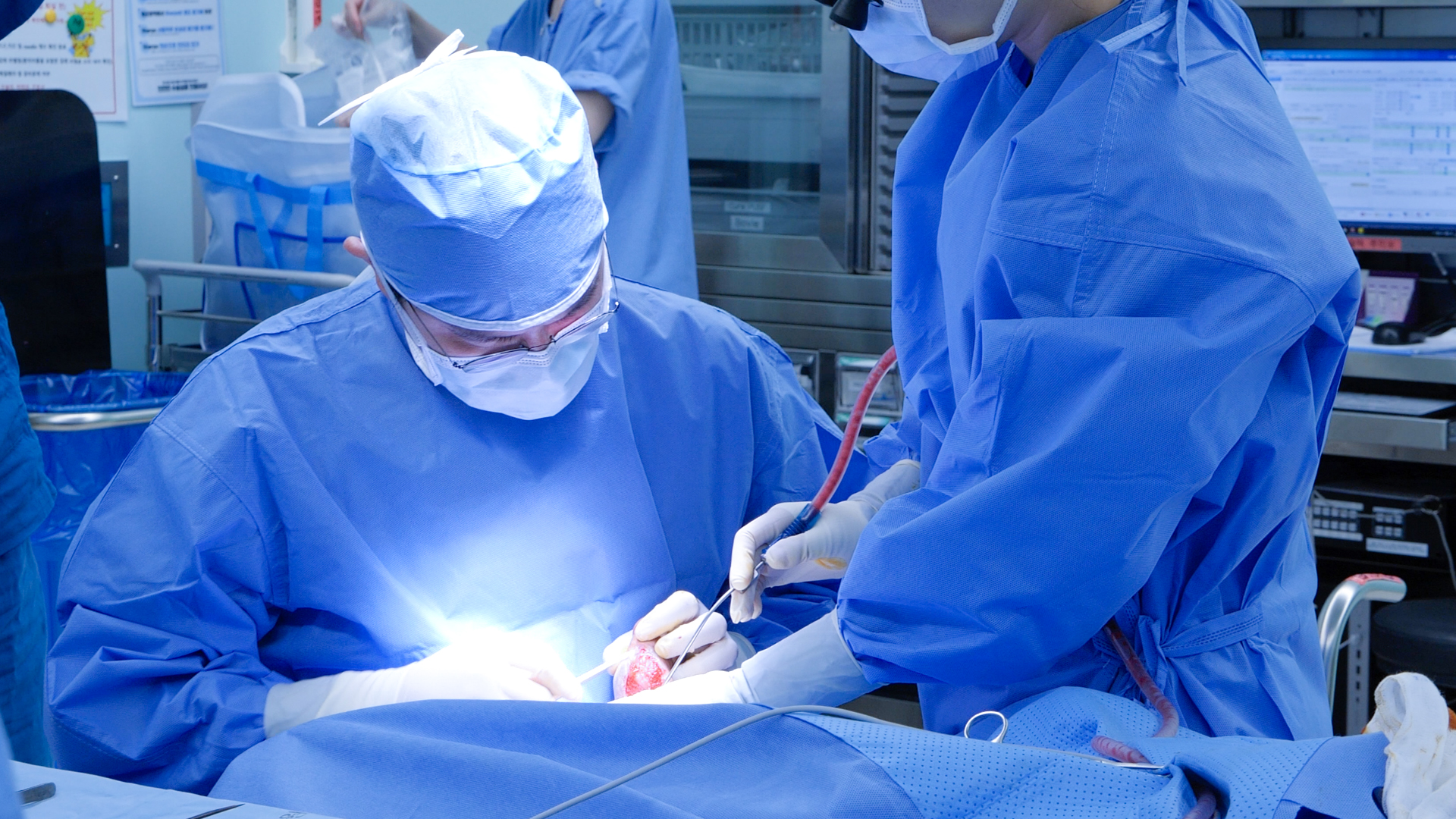
The AI Hyper-Personalized Organ Regeneration Platform is designed to regenerate tissues and treat incurable chronic diseases through cutting-edge biomedical innovation.
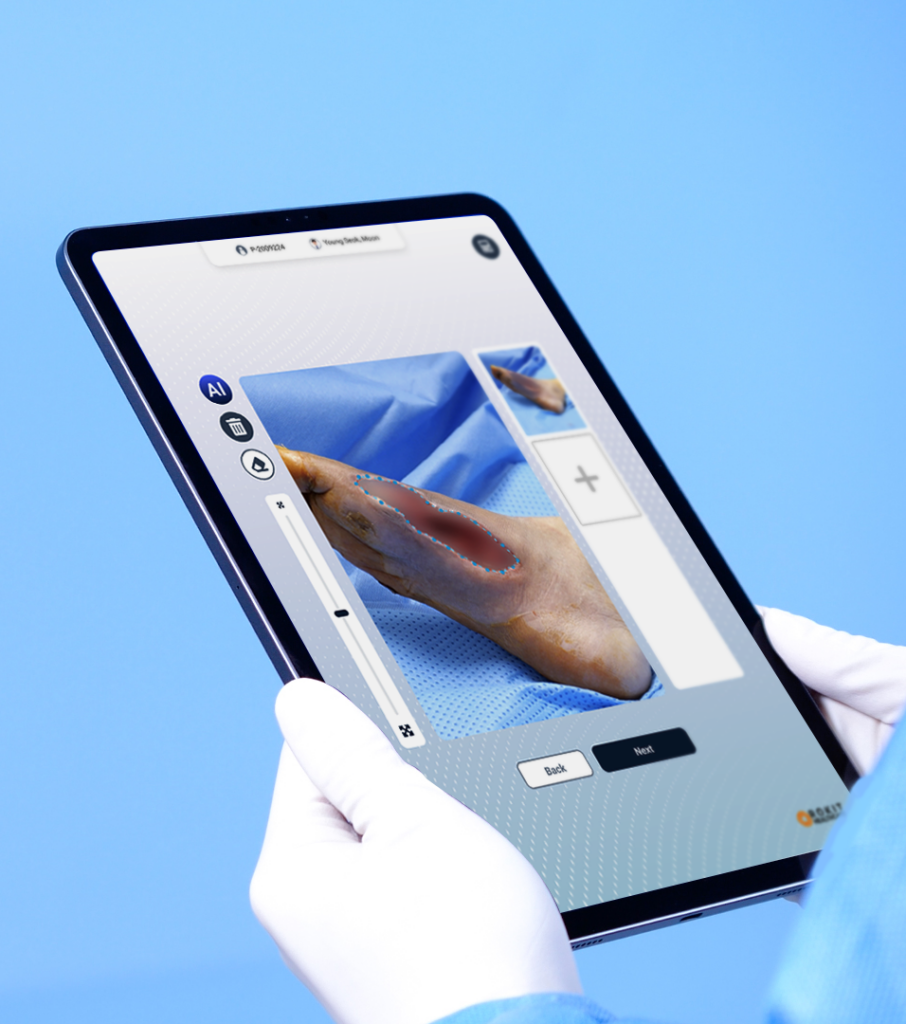
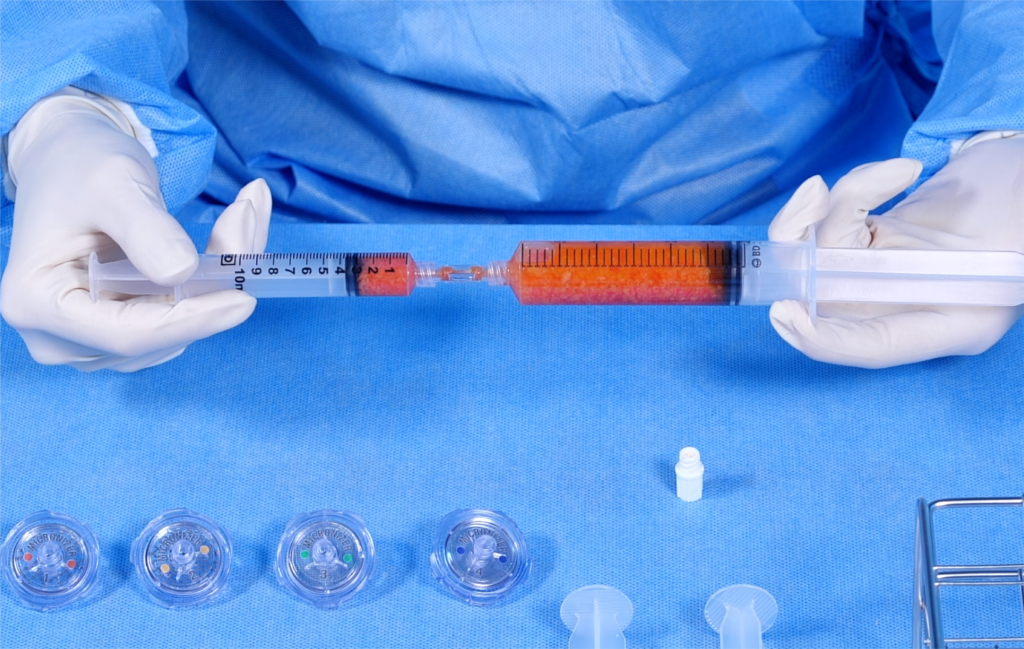
As a first-mover in the field, this pioneering platform integrates artificial intelligence (AI), a 3D bioprinter, and a one-time-use medical device kit to deliver personalized regenerative therapies.
Developed through a multidisciplinary convergence of advanced technologies, the platform is already being installed and actively utilized in operating rooms of leading global hospitals, marking a new era in precision medicine and organ regeneration.

Get in touch
Pages
Follow us


